What are stem cells?
Stem cells have the potential to revolutionize the field of medicine. These cells possess the unique ability to renew themselves and transform into specialized cell types. Their versatility offers a wide range of applications, such as disease modeling and the regeneration of damaged tissues or organs.
Where do stem cells come from?
Stem cells can be derived from different sources, which include those from embryonic, adult, and induced origin. The diversity of stem cell types, each with its developmental profile and origin, enables their tailored application to patients with specific diseases and ethical perspectives.
- Embryonic Stem Cells (ESCs):
ESCs are derived from the inner cell mass of the blastocyst-stage embryo. They are pluripotent, meaning they can differentiate into any cell type in the human body. ESCs have been extensively studied for their potential in regenerative medicine due to their ability to generate various cell lineages. However, their use is a subject of ethical debate due to the destruction of embryos required for their isolation.
- Adult Stem Cells:
Adult stem cells are found in different tissues and organs throughout the body. These stem cells are multipotent or unipotent, meaning they can differentiate into a limited range of cell types specific to the tissue from which they are derived. Adult stem cells include hematopoietic stem cells (HSCs) found in the bone marrow and mesenchymal stem cells (MSCs) found in various tissues such as bone, adipose tissue, and muscle. HSCs can differentiate into various specialized cell types found in the blood, such as red blood cells, white blood cells, and platelets. MSCs can differentiate into various cell types of mesodermal origin, such as bone cells (osteocytes), cartilage cells (chondrocytes), and fat cells (adipocytes). They are also known for their immunomodulatory properties, as they can regulate the immune response by suppressing or facilitating inflammation
- Induced Pluripotent Stem Cells (iPSCs):
Induced pluripotent stem cells are generated by reprogramming adult cells, such as fibroblasts or blood cells, to a pluripotent state. This reprogramming is achieved by introducing specific transcription factors into the cells. iPSCs have properties similar to embryonic stem cells and can differentiate into various cell types. They have revolutionized the field of regenerative medicine by providing a potential source of patient-specific stem cells with fewer ethical concerns compared to ESCs.
- Perinatal stem cells
Perinatal stem cells refer to stem cells obtained from tissues surrounding the time of birth, such as the umbilical cord, placenta, and amniotic fluid. Most of these are MSCs. These stem cells offer distinct advantages compared to other types, including accessibility, ethical acceptability, and robust proliferative capacity. Hematopoietic stem cells derived from umbilical cord blood (also known as Wharton’s jelly) exhibit multipotent differentiation capabilities, and contains a rich combination of growth factors, cytokines, hyaluronic acid, and extracellular vesicles found in higher quantities compared to other biologics. Amniotic fluid stem cells, obtained from the fluid surrounding the fetus, possess the ability to differentiate into diverse tissue types and exhibit immunomodulatory properties. Research and clinical trials are required to comprehensively explore the therapeutic potential of perinatal stem cells, but their unique properties and wide availability make them a promising avenue for future advancements in regenerative medicine.
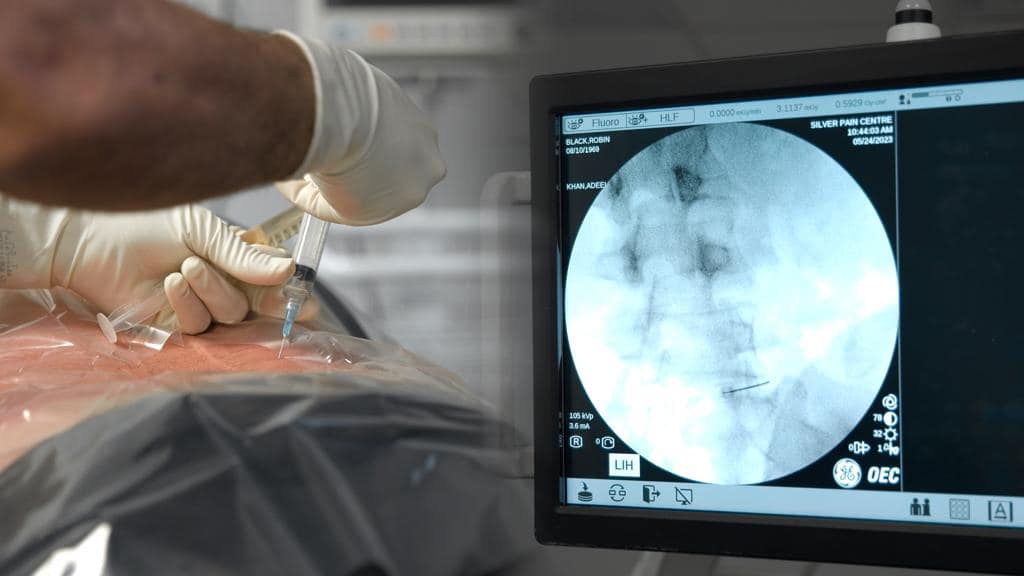
What is the current use of stem cells?
In Canada and the United States, stem cells are being used to create disease models for genetic disorders, neurodegenerative diseases, and drug testing. These models enable researchers to understand disease mechanisms better and develop targeted therapies.
Clinically, stem cells in the United States and Canada are primarily used for hematopoietic (blood) stem cell transplantation to treat disorders of the blood or immune system. In Canada, stem Health Canada regulates cell therapy, and the Canadian Blood Services operates a national stem cell registry and facilitates matching donors with patients. In the United States, the regulatory authority for overseeing regenerative medicine products lies with the United States Food and Drug Administration (FDA).
In Japan, stem cells have also been approved to treat blood disorders; but they can also be used for spinal cord injuries, age-related macular degeneration, and myocardial infarction. At present, insurance providers extend coverage to encompass cell transplantation therapies used for repairing damaged skin, articular cartilage, and stroke-related conditions.
Regenerative medicine is more widely used and advanced in Japan because they have implemented rigorous regulatory measures to govern regenerative medicine and stem cell therapies to ensure their safety and efficacy. Specifically, the Pharmaceuticals and Medical Devices Agency (PMDA) has played a vital role in establishing guidelines and regulations in this field. The regulations set strict requirements for manufacturers, including comprehensive data submission on the manufacturing process, characterization of the cellular product, and results from preclinical and clinical studies. Japan’s “Sakigake Designation System” expedites the review process for innovative medical products, including regenerative medicine, addressing unmet medical needs. This accelerated system has attracted researchers, investors, and companies, fostering advancements in the field within Japan. Overall, Japan’s robust regulatory framework demonstrates a commitment to promoting the safe and effective use of regenerative medicine and stem cell therapies, benefiting patients and advancing regenerative science.
Conclusion
Stem cells hold tremendous promise in the field of regenerative medicine and beyond. Their ability to self-renew and differentiate into specialized cell types makes them invaluable for various applications. While current uses in Canada and the United States are relatively limited, regenerative medicine technology, including stem cells, thrives in Japan. This is because the Japanese government has implemented stringent regulations and guidelines to ensure the safety and efficacy of regenerative therapies. Canada lacks a comparable regulatory system for regenerative medicine, which hinders its progress in the field. To catch up, Canada needs to develop a similar framework to Japan to promote innovation, attract investment, and provide patients with access to advanced regenerative medicine treatments.
For this reason, Eterna Health collaborates with clinics worldwide to provide our patients with cutting-edge technology, irrespective of national borders.