What Are Exosomes?
Exosomes are small, membrane-bound vesicles that are released by cells and contain various biomolecules, such as proteins, nucleic acids, and lipids. They play a role in intercellular communication and can be found in various biological fluids, including blood, urine, and breast milk. Exosomes are also being used for several medical applications, disease diagnosis and regenerative treatments such to reduce inflammation, oxidative stress, increase blood flow, and slow down general degeneration.
What Are Vesicles?
Vesicles are small, membrane-bound sacs that are found within cells. They can be formed by the cell’s membrane as well as by intracellular organelles, such as the endoplasmic reticulum, Golgi apparatus, and mitochondria. Vesicles play important roles in intracellular transport, storage, and secretion of molecules such as proteins, lipids, and other small molecules. They also play a key role in intercellular communication by facilitating the transfer of molecules between cells, as in the case of exosomes.
What Are Extracellular Vesicles?
Extracellular vesicles (EVs) are a type of vesicle that is released by cells into the extracellular space. They are similar in structure and composition to intracellular vesicles, but they are released into the extracellular environment instead of being retained within the cell.
EVs include various types of vesicles, such as exosomes, microvesicles, and apoptotic bodies, that differ in their size, biogenesis, and cargo. EVs are involved in a variety of physiological and pathological processes, such as intercellular communication, immune response, and cancer progression. They also have potential as diagnostic and therapeutic tools due to their ability to transfer bioactive molecules between cells.
What Are Microvesicles?
Microvesicles are small, membrane-bound vesicles that are shed from the surface of cells. They are similar in size and composition to exosomes, but they are formed by a different mechanism. Microvesicles are generated by the outward budding and fission of the cell’s plasma membrane, whereas exosomes are formed within the endosomal pathway of the cell. Microvesicles have been shown to contain various biomolecules, such as proteins, lipids, and nucleic acids, and they have been implicated in a variety of physiological and pathological processes, including intercellular communication, coagulation, and inflammation. Like exosomes, microvesicles also have potential as diagnostic and therapeutic tools due to their ability to transfer bioactive molecules between cells.
Are All Exosomes The Same?
In short, No. Exosomes can vary in their size, composition, and function, depending on the type of cell from which they are released and the physiological or pathological state of the cell. Exosomes can differ in their protein, lipid, and nucleic acid content, as well as their cargo of functional molecules, such as enzymes, growth factors, and microRNAs. The cargo of exosomes is thought to reflect the functional state of the cell that releases them, and exosomes can therefore be used as a source of biomarkers for disease diagnosis or as a tool for therapeutic intervention. Furthermore, exosomes from different cell types have been shown to have different functions, such as promoting or inhibiting immune responses or modulating tumor growth and metastasis. Therefore, it is important to carefully characterize the exosomes of interest in order to fully understand their properties and potential applications.
Where Does Eterna Get Its Exosomes?
We have partnered with Neobiosis to use their purified amniotic fluid (PAF) exosome product. Amniotic fluid flowable tissue differs in source material, quality of manufacturing, sterilization technique, size, concentration, protein and RNA concentration and potential risk of fungal and bacterial contamination.
Our Purified Amniotic Fluids are refined, which produces a crystal-clear product, never pink (red blood cell contamination) and never cloudy (suggestive of contamination with microvesicles and apoptotic bodies, which may inhibit the regenerative process or induce inflammation, compromising research and clinical trial outcomes). Neobiosis PAF are minimally manipulated, twice sterile filtered, and never UV irradiated.
While some labs attempt to sterilize AF with UV irradiation, Neobiosis scientists understand that irradiation (UV, e-beam, Gamma, etc) destroys RNA and is not an accepted practice for sterilizing fluids. Only filtration with validated, filter- isolation equipment is sufficient to ensure product quality and sterility.
What Are Exosomes Used to Treat?
Exosomes are being used as a therapeutic tool for a variety of conditions. They have been investigated for their ability to deliver drugs or therapeutic molecules, as well as for their ability to promote tissue regeneration and modulate the immune response. Some of the conditions that exosomes are being studied to treat include:
- Cancer: Exosomes are being investigated as a way to deliver drugs or genetic material to cancer cells or to stimulate the immune system to attack cancer cells.
- Neurological disorders: Exosomes may be able to cross the blood-brain barrier and deliver therapeutic molecules to the brain, making them a potential treatment for neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease.
- Cardiovascular disease: Exosomes may be able to promote the regeneration of damaged tissue and improve blood vessel function, making them a potential treatment for cardiovascular diseases, such as heart attack and stroke.
- Inflammatory and autoimmune disorders: Exosomes have been shownto have anti-inflammatory properties and may be able to modulate the immune response, making them a potential treatment for inflammatory and autoimmune disorders, such as rheumatoid arthritis and multiple sclerosis.
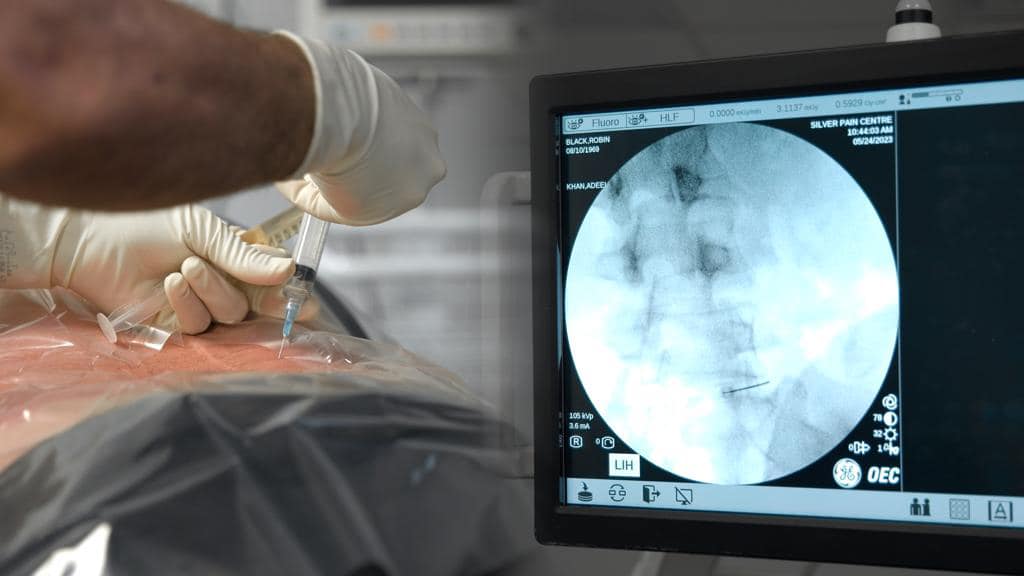
- Musculoskeletal disorders: Ultrasound or Fluoroscopic guided exosome injections for osteoarthritis, tendinopathies and chronic nerve or disc pain have yielded great results. There are many studies indicating the benefits with exosomes for treating chronic pain with no significant adverse events.
- Aging.
Perinatal exosomes have gained attention for their potential to impact the hallmarks of aging at a cellular level. The hallmarks of aging are a set of interconnected cellular and molecular processes that contribute to aging and age-related diseases. Let’s explore how perinatal exosomes can influence these hallmarks:
- Genomic instability: Genomic instability refers to DNA damage and mutations that accumulate over time. Perinatal exosomes can contain molecules, such as DNA repair proteins and antioxidants, which can help maintain genomic stability and repair damaged DNA. By delivering these components to recipient cells, perinatal exosomes may enhance DNA repair mechanisms and mitigate genomic instability.
- Telomere attrition: Telomeres are protective caps at the ends of chromosomes that shorten with each cell division. Perinatal exosomes can potentially modulate telomere maintenance by delivering telomerase or factors that regulate telomerase activity. This may slow down telomere shortening and extend the replicative lifespan of cells, counteracting telomere attrition. Developmental signals involved in tissue growth and repair, such as Wnt/β-catenin and Notch pathways, can upregulate telomerase activity in stem cells and progenitor cells. Various growth factors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF), can activate signaling pathways that stimulate telomerase activity. These growth factor signals often involve the activation of downstream pathways, including the PI3K/Akt and MAPK/ERK pathways, which can upregulate telomerase activity.
- Epigenetic alterations: Epigenetic modifications, such as DNA methylation and histone modifications, play a crucial role in gene expression regulation. Perinatal exosomes may carry regulatory molecules, including microRNAs (miRNAs) and other epigenetic regulators, that can influence the epigenetic landscape of recipient cells. By modulating gene expression patterns, perinatal exosomes could potentially reverse or attenuate age-associated epigenetic alterations.
- Loss of proteostasis: Proteostasis refers to the maintenance of proper protein folding, quality control, and degradation. Perinatal exosomes can transport heat shock proteins (HSPs) and other chaperones that aid in protein folding and prevent protein misfolding and aggregation. By promoting proteostasis, perinatal exosomes may help mitigate the accumulation of misfolded proteins and maintain cellular function.
- Cellular senescence: Cellular senescence is a state of irreversible cell cycle arrest that contributes to aging. Perinatal exosomes have been shown to modulate cellular senescence by delivering factors that can either promote or suppress senescence-associated pathways. For example, they may carry miRNAs that target senescence-inducing genes or factors that enhance the regenerative capacity of senescent cells. Exosomes affect the JAK-STAT pathway . The JAK-STAT pathway can regulate cell cycle progression by affecting the expression of various cell cycle-related genes, including cyclins and CDK inhibitors. Therefore exosomes can help with cell cycle progression, thereby preventing cellular senescence.
- Altered cellular metabolism: Cellular metabolism undergoes changes with aging, including mitochondrial dysfunction and altered nutrient sensing pathways. Perinatal exosomes can transport metabolic regulators, such as miRNAs or metabolites, that influence cellular metabolism and mitochondrial function. By delivering these factors, perinatal exosomes may help restore or improve cellular metabolic processes.
- Stem cell exhaustion: Stem cell function declines with age, leading to diminished tissue repair and regeneration. Perinatal exosomes can interact with stem cells and influence their proliferation, differentiation, and regenerative potential. By delivering regenerative factors and signaling molecules, perinatal exosomes may rejuvenate or support stem cell populations, potentially mitigating stem cell exhaustion.
Overall, perinatal exosomes have the potential to impact multiple hallmarks of aging at a cellular level by delivering various bioactive molecules to recipient cells. Exosome-based therapeutics and its impact on aging and disease is still an area of active research, and further studies are needed to fully understand and harness their potential in combating aging and degenerative diseases. A pragmatic approach to fight aging and disease that is now implemented by many NFL & NBA athletes is to do IV exosomes every 6 months. At Eterna, we are the only clinic in Canada offering Platelet-rich plasma derived exosomes using our proprietary ultracentrifugation method.